
Abdullah Al Mamun Khan1*, Muhammad Jahangir Alam2, Parveen Shahida Akhtar3
1Department of Medical Oncology, Shaheed Suhrawardy Medical College Hospital, Dhaka, Bangladesh.
2Medical Oncology, National Institute of Cancer Research and Hospital, Dhaka.
3Professor of Medical Oncology, Shanti Cancer Foundation, Mohammadpur, Dhaka, Bangladesh.
*Corresponding Author Abdullah Al Mamun Khan, Department of Medical Oncology, Shaheed Suhrawardy Medical College Hospital, Dhaka, Bangladesh.
Received: May 21, 2023
Accepted: June 02, 2023
Published: July 10, 2023
Citation: Abdullah Al Mamun Khan and, Muhammad Jahangir Alam and Parveen Shahida Akhtar. (2023) “Five years survival rate of adolescents and young adults (AYA) Patients from GIT cancer: A prospective fixed cohort study in Bangladesh”, J Clinical Oncology and Cancer Research, 1(1); DOI: http;//doi.org/009.2023/1.1004.
Copyright: © 2023 Abdullah Al Mamun Khan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background:
Survivors of adolescent and young adult cancers (AYA) often live 50 to 60 years beyond their diagnosis. Overall cancer incidence increased in all AYA age groups during the most recent decades but a few clinical and epidemiological data from developing countries. Gastrointestinal Tract (GIT) cancer is a leading group of AYA cancer that may manageable if diagnosed early. This study was aimed to find out the 5 year OS rate of AYA patients from GIT cancer.
Methods: The study was a prospective fixed cohort study. Study period was 2016 to 2021.
Results: Of total 96 patients, male and female ratio was 1.91:1. After follow up of 5 years, it was documented that 48(50%) patients were drop out from follow up. Among the remaining 50% patients, 38(79.17%) patients were died and 10(20.83%) patients were alive. Alive male were 7(70%), female were 3(30%) and the ratio was M:F=2.33:1. Among the survival, highest were in colon cancer 6(60%) followed by stomach cancer 2(20%%), gall bladder cancer 1(10%) and pancreatic cancer 1(10%). The leading age group of affected was 35 to 39 years, 43(44.79%) followed by 30-34 years group, 25(26%). It was documented that colon cancer was the highest occurrences, 48(50%) followed by stomach cancer, 23(23.95%). 26(27.10%) patients were presented with metastasis during diagnosis. 8(8.33%) AYA patients were with comorbidities. 33(34.38%) patients had a bad personal habit including 26(27%) were smoker and 7(7.3%) were betel nut chewer. None had insurance coverage and 92% were dependent for treatment cost to their family or others.
Conclusion:
Evidence-based guidelines for AYA have not been developed for evidence-based late effects screening and care guidelines for AYA cancer patients. The high death occurrence dangerously alarming for Bangladesh as youth are majority of total population. So methods should be searched to reduce the AYA death occurrences.
Introduction:
The discipline of adolescent and young adult oncology (AYAO) is an evolving field that has begun to be defined only within the last decade. The increasing focus over the last 15 years on the outcomes, unique challenges of care and distinct biology of young adult cancers is beginning to stimulate interest in the development of clinical programs specific to the care of AYAs. Cancer is regarded as a disease of older adults. Little is known and reported in literature about the incidence and patterns of this disease in adolescent and young adults (AYAs). This population poised between children and adults has been called the “lost tribe” [1].
Adolescent and young adult (AYA) cancer patients, aged 15–39 years at primary cancer diagnosis, form a distinct, understudied, and underserved group in cancer care. [2] Overall cancer mortality declined during 2008 through 2017 by 1% annually across age and sex groups, except for women aged 30 to 39 years, among whom rates were stable because of a flattening of declines in female breast cancer. [3]
There is no study in Bangladesh with AYA malignancies in adolescents and young adults. There are limited studies and literatures are available from developed countries .The annual number of AYA cancers, although small, will constitute a significant burden .This diagnosis disrupts the normal trajectories of development including physical, psychological, social and life goals related to family and careers. For this reasons, cancer in AYA merits attention.
Progress in reducing cancer morbidity and mortality among AYAs could be addressed through more equitable access to health care, increasing clinical trial enrollment, expanding research, and greater alertness among clinicians and patients for early symptoms and signs of cancer. Further progress could be accelerated with increased disaggregation by age in research on surveillance, etiology, basic biology, and survivorship.
Early detection of the disease by recognizing signs and symptoms that appeared first will be beneficial for the patients with AYA tumor as prognosis differs with the stage of the disease. Although cancer is the second leading cause of death in AYA (12% of death), it is still relatively uncommon. The incidence of cancer is increasing. Fortunately, with modern aggressive multidisciplinary therapy in developed countries, 5-year survival for this group with cancer exceed 75% [4].
Many cancers, including lymphoma, leukemia, sarcomas, melanoma, GI stromal tumor, breast cancer, and colon cancer ; the epidemiology and cancer biology differ in AYA cancers compared with younger children and older adults [5-6]. Recent studies have found differences in outcomes for AYA cancers in certain cancers depending on whether they were treated on pediatric or adult protocols. Generating and delivering treatment care plans for the AYA population requires awareness of and sensitivity to these issues. The recommendation of the AYAO PRG is “to provide education, training, and communication to improve awareness, prevention, access, and quality of care for AYAs.”[6-7]
A need to examine survival trends of individual cancers in older adolescents and young adults (AYA) is prompted by overall survival trends that have indicated a lack of progress in survival improvement for AYAs compared with both younger and older cancer patients. In 19 of 21 cancers for which a comparison of survival by gender is feasible, AYA males had a worse survival rate than females [7-9]. AYA patients with cancer, aged 15 to 39 years, have not shown the same improved survival as older or younger cohorts [8-10]. The age limit of this study was 15 to 39 years. Our objective was to study the descriptive epidemiology of cancers in AYA age group at NICRH and to compare this with the available data in other countries literature. There are 72006601.45(44.2%) people under this 15-39 years age group [9-12]. They are in vital age group not only for their own development but also for their country.
Material and Methods:
The study was an observational study. All histologically confirmed GIT cancer patients aged 15-39 years who completed treatment under medical oncology department in National Institute of Cancer Research and Hospital (NICRH) from Jan.2016 to Dec.2016(12 months) were enrolled for this study. Of them 96 patient were included for the study. Their follow up were taken over phone or departmental follow up registries up to 2021 marking completion of five years from registry individually. The sample was collected by purposive sampling technique. Prior to commencement of this study, the research protocol was approved by the respective authority (RRC & EC) of NICRH, Dhaka. Consent were taken with written documents. After 5 years of complete their treatment, each patient were tried to take follow up over phone and arranged according to patient’s condition like alive or death or untraceable. The follow up patients were arranged according to Barch10 classification at 5 year interval.
Results:
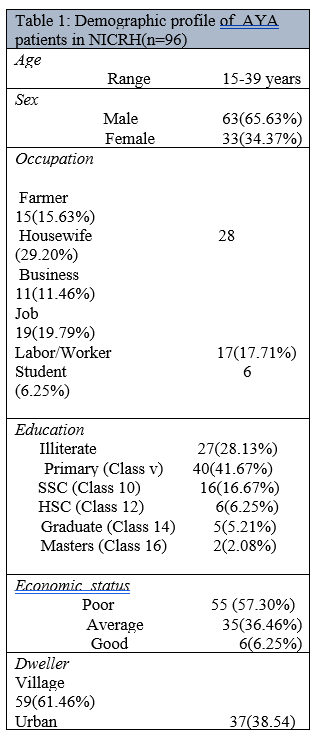
Of total 96 patients, male was 63 and female was 33 in number and their ratio was 1.91:1. Patients demographic profiles are detailed in table 1.
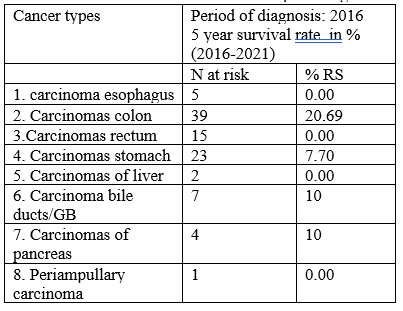
After follow up of 5 years, it was documented that 48(50%) patients were drop out from follow up and we don’t know whereas they were alive or not because their contact numbers were off or had not. Among the remaining 50% patients, 38(79.17%) patients were died and 10(20.83%) patients were alive. Alive male were 7(70%), female were 3(30%) and the ratio of survival was M:F=2.33:1. Among the survivals, highest survival rate were in colon cancer 6(60%) followed by stomach cancer 2(20%), gall bladder cancer 1(10%) and pancreatic cancer 1(10%) and detailed in table 2.
Table 2: Relative survival rate in percentage
The leading age group of affected was 35 to 39 years, 43(44.79%) followed by 30-34 years group, 25(26%); 25-29 years group was 13(13.54%) and 20-24 years group was 11(11.46%) and least affected was 15-19 years age group, 6(6.25%). It was documented that colon cancer was the highest occurrences, 39(40.63%) followed by stomach cancer, 23(23.96%), rectal cancer, 15(15.63%); gall bladder cancer, 7(7.29%); Esophageal cancer, 5(5.21%) and pancreatic cancer, 4(4.17%) and detailed in table 3.
|
Age group |
Year of diagnosis : 2016, 5 year survival rate in %(2016-2021) |
||||||
|
|
Male(M) affected |
M alive |
M died |
Female(F) Affected |
F alive |
F died |
Out of follow up of both sex |
|
15-19 years. |
5 |
1 |
1 |
1 |
0 |
1 |
3 |
|
20-24 years. |
6 |
1 |
4 |
4 |
0 |
1 |
4 |
|
25-29 years. |
10 |
1 |
4 |
2 |
0 |
0 |
7 |
|
30-34 years. |
13 |
1 |
4 |
12 |
1 |
5 |
14 |
|
35-39 years. |
29 |
3 |
12 |
14 |
2 |
6 |
20 |
|
Total= |
63 |
7 |
25 |
33 |
3 |
13 |
48 |
Table 3: Survival rate according to age group.
26(27.10%) patients were presented with metastasis during diagnosis who were not alive and alive AYA had no metastasis during diagnosis. 12(12.5%) patients were presented with locally advanced diseases initially.
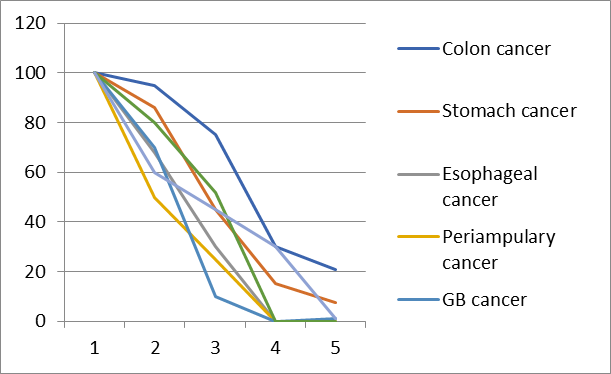
Figure 1: 5 years survival rate of GIT cancer in AYA patients.
8(8.33%) AYA patients were with comorbidities and with comorbidities AYA patients all were died. 33(34.38%) patients had a bad personal habit including 26(27%) were smoker and 7(7.3%) were betel nut chewer and none of them were alive. None had insurance coverage and 92% were dependent for treatment cost to their family or others.
According to performance status (PS), 44(45.83%) patients were with PS 2; 23(23.96%) were PS 3; 17(17.71%) AYA were with PS 1; 4(4.17%) patients were with PS 4 and only 8(8.33%) AYA were with PS 0. Some of AYA patients made delay to start treatment like 16(16.66%) patients delayed 30 days, 14(14.58%) patients delayed 60 days, 6(6.25%) patients delayed 90 days, 8(8.33%) patients delayed 120 to 180 days and 3(3.12%) patients delayed over 180 days to start their specific treatment . 59(61.46%) AYA lived in rural area and 37(38.54%) lived in urban area. 55(57.30%) patients were poor, 35(36.46%) patients were average socio-economical condition and only 6(6.25%) were in good socioeconomic condition. 27(28.13%) patients were illiterate, 40(41.66%) AYA were educated up to primary (class 5), 16(16.66%) were educated up to SSC (class 10), 6(6.25%) were educated up to HSC (class 12) and 5(5.21%) were Graduate(class14) and only 2(2.08%) patients were master degree(class16) holder. 28(29.17%) AYA were house wife by profession followed by 19(19.79%) were job holder, 15(15.63%) were farmer, 17(17.71%) were worker, 11(11.46%) were business men and only 6(6.25%) AYA were student. None had insurance coverage and 92% were dependent for treatment cost to their family or others.
|
|
n |
SEC |
Metastasis |
Comorbidity |
P/H |
Delay |
PS |
Dweller |
|
Alive |
10 |
P=7(70%) A=3(30%) |
Nil |
Nil |
Nil |
Max. 30 days |
Max.2 |
R=5(50%) U=5(50%) |
|
Death |
38 |
P=12(31.58%) A=14(36.84%) |
13(34.21%) |
5(13.16%) |
S=13(34.21%) B=5(13.16%) |
Max. 2 years |
Max.4 |
R=16(42.12%) U=12(31.58%) |
Table 4: Comparison of contributing factors between death and alive
N=case, SEC=Socioeconomic condition, P/H=Bad personal habit, Max=Maximum, PS=performance status, P=poor, A=average income, S=smoker, B=betel nut chewer, R=rural, U=urban
Conclusion:
This high death occurrence dangerously alarming for developing countries as youth are majority of total population in developing countries. This study showed that metastasis during diagnosis, comorbidities and making delay to start treatments were indicative factors for survival. Residence were an important foctor also because urban patients got maximum benefit regarding treatment and facilities available here than rural. Thats why death rate more in villages. Socio-economical status was not a factor for survival.
So methods should be searched to reduce the AYA death occurrences. AYA cancer patients varies among different countries across the world. More research in this field will help us understand the needs of this unique reproductive young population of Bangladesh. Cancer in AYA is an important problem that has gone unrecognized or was only a peripheral concern among numerous research, medical and health services payer, patient support and advocacy, funding, and cancer surveillance constituencies, as well as healthy teenagers and young adults who do not know they are at risk for cancer.
Conflict Of Interest: No